Current A3 Programs
0
Phase I
0
Phase II
0
Phase III
Commercial Programs 1
How Can Amsphere™ A3 Help Me?
Besides an outstanding high capacity, Amsphere™ A3 has an overall improved process robustness, flow characteristics, optimized impurity removal, productivity and resin lifetime.
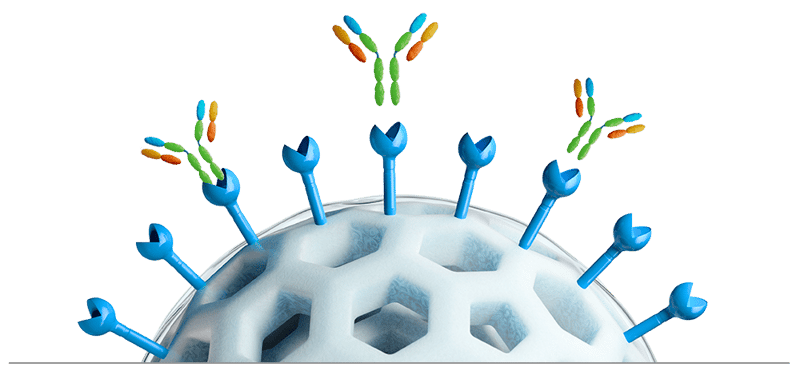
| Protein A Ligand High DBC via controlled conformation and orientation High alkaline stability from protein engineering |
Surface modification Low HCP levels by surface hydrophilization |
Base bead formulation High DBC at high flow rate Good pressure and flow properties via rigid crosslinking |
Resources
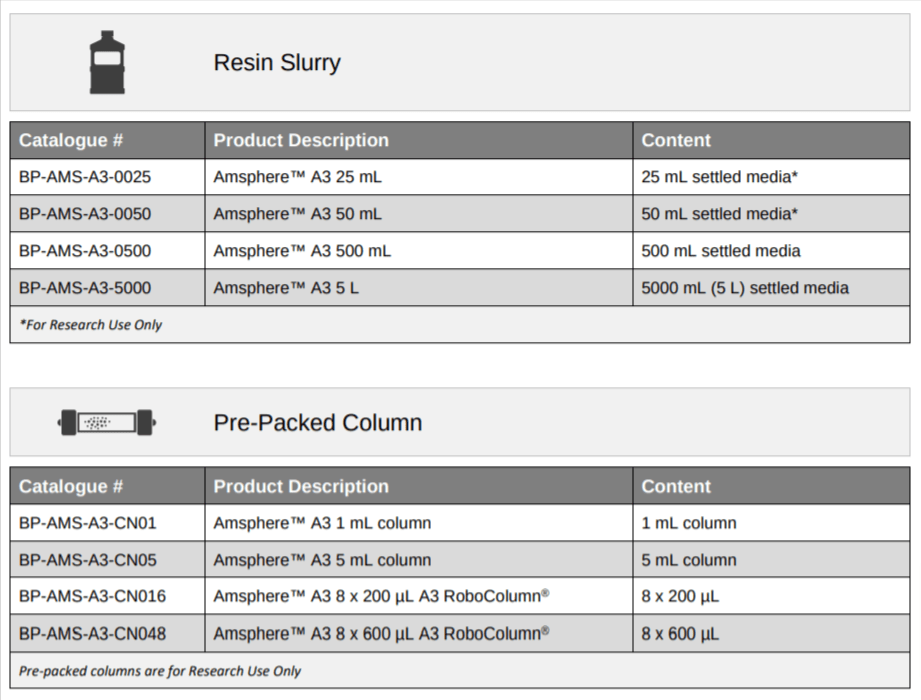
Catalog Numbers & Product Sizes
Why use Amsphere™ A3?
-
Outstanding Dynamic Binding Capacity
A highly cross-linked bead structure and controlled attachment of the protein A ligand results in a superior dynamic binding capacity.
At higher flow rates the semi-rigid bead structure allows to achieve up to 50% higher DBC compared to the market standard product at low to moderate residence times of 2-6 minutes..jpg?width=800&name=dynamic_binding_capacity3%20(1).jpg)
-
Impurity Clearance At Industry Standard
The surface treatment of the polymeric base matrix resulted in low HCP, DNA, virus and aggregate levels that are very similar to hydrophilic backbones such as agarose.
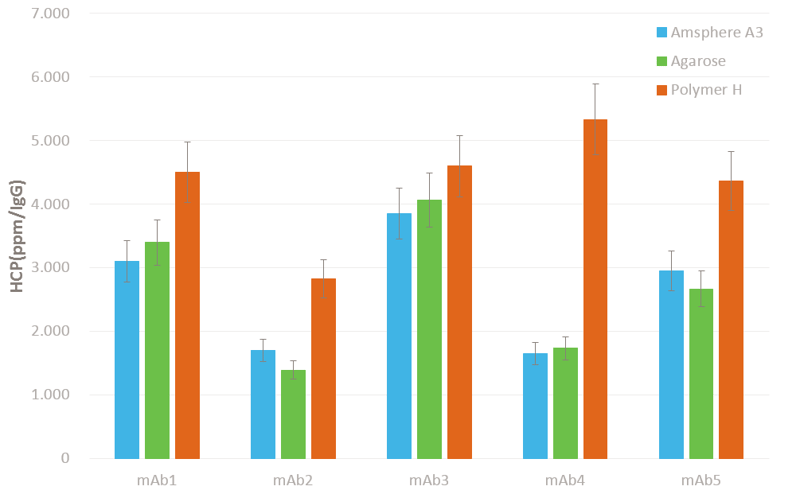
-
Superior Caustic Stability
Amsphere™ A3 can resist up to 300 cycles with 0.1 M sodium hydroxide as CIP solution (15 minutes of contact time) while maintaining a dynamic binding capacity above 90%. In addition, 0.5 M sodium hydroxide can be used for at least 100 cycles (15 minutes of contact time).
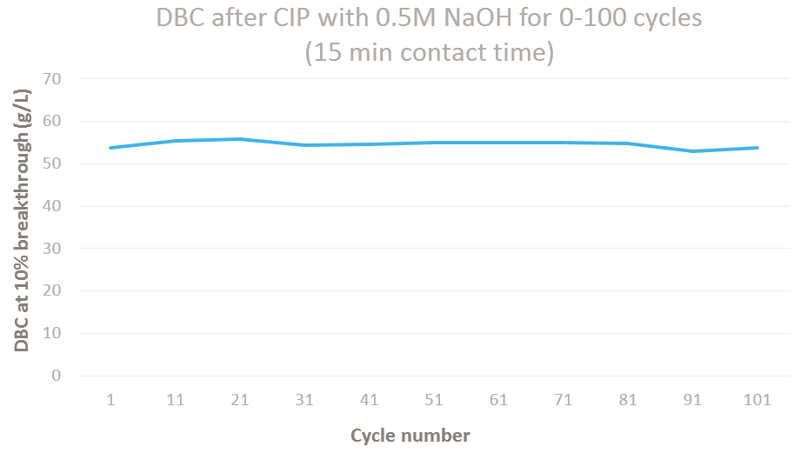
-
Regulatory Compliance
- Amsphere™ A3 resin manufacturing, testing, packaging, storage, and distribution are all done in accordance with ISO9001 standards and certified.
- All raw materials used in the manufacturing process of Amsphere™ A3 are evaluated and free of animal-derived substances and are considered safe regarding BSE/TSE risk.
- Amsphere™ A3 resin is used in the biopharma industry for the GMP manufacturing process of clinical and commercially approved biologics.
- The Regulatory Support File is available under a Confidential Disclosure Agreement (CDA).
- Amsphere™ A3 resin manufacturing, testing, packaging, storage, and distribution are all done in accordance with ISO9001 standards and certified.

Protein A Mix-N-Go™ ELISA for Amsphere™ A3 Ligand
Ready-to-use pre-packed RoboColumn®s (200 – 600 µL) and small scale 1 mL and 5 mL columns are available for screening purposes and process development. Larger formats including GMP compliant pre-packed columns are also available on request.
An Amsphere™ A3 ligand specific ELISA kit is available through Cygnus Technologies. Please click here to order the kit.
Why Amsphere A3 is an Outstanding Choice for Antibody Fragment & FC-Fusion Purification
Universal Affinity Resin for
Simplify Your Supply Chain
& Minimize Costs
Excellent
Impurity Clearance
.png?width=420&height=420&name=Untitled%20design%20(47).png)
Presenter: Beatriz Catalao
Application Engineer, JSR Life Sciences
.png?width=284&height=68&name=JSR%20LS%20White%20Horizontal%20Logo%202019OCT62019%20(1).png)
OnDemand Webinar
Packing of Protein A resins in lab-, pilot-and large-scale columns: How to achieve a "right first time"- PART 1
This video webinar, presented by Beatriz Catalao of JSR Life Sciences, is on the topic of column packing of protein A resins in lab, pilot, and large-scale columns. Beatriz provides a quick introduction to Amsphere A3, a protein A resin, and highlights the column packing preparation requirements to achieve a successfully packed bed.
Beatriz also mentions the high binding capacity, alkali stability, and impurity removal capabilities of Amsphere A3, emphasizing its added value for default batch and next-generation intensified bioprocessing. Additionally, Beatriz mentions the availability of regional application support, on-site packing support, packing training, and experimental support.
Throughout the webinar, various methods and factors for determining slurry concentration and compression factor are explained, along with guidelines for selecting the right column and bed supports. The video also discusses the recommended compression factors for different column diameter ranges and provides flow rate ranges for different column sizes. Finally, it presents guidelines for resin slurry handling and discusses suitable and unsuitable pump options for transferring the slurry.
This is part one of a webinar series and focuses on the column packing process preparations, with a second webinar planned to cover column packing and evaluation.