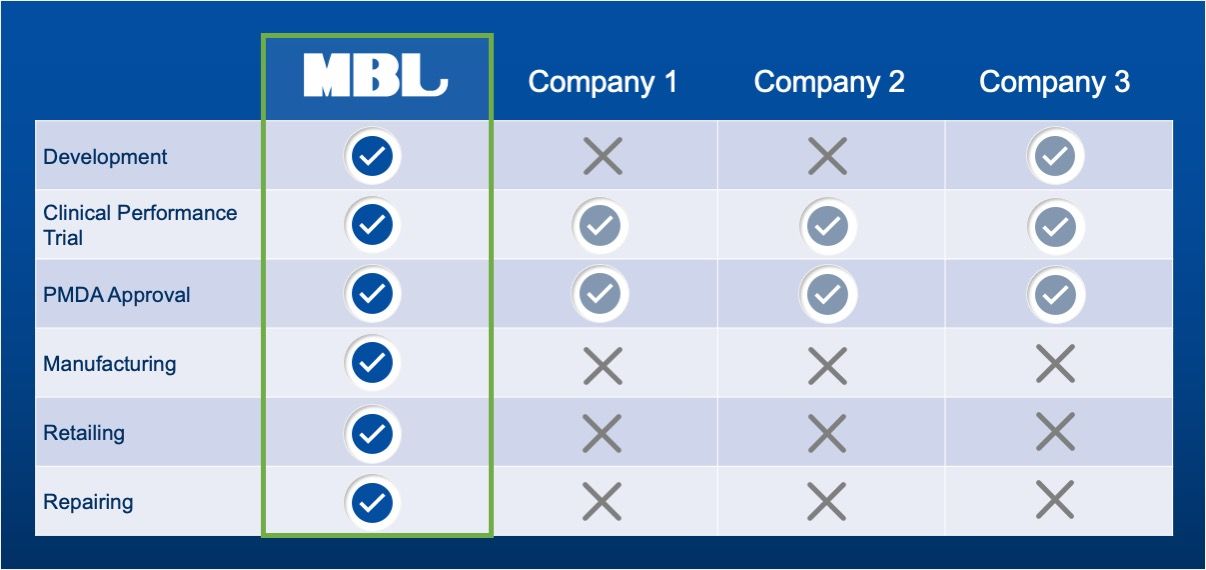
One Stop Shop
MBL has all the required business licenses for medical device manufacturing, marketing, and sales of IVD/CDx products and repairing of medical devices in Japan. We are uniquely positioned to support foreign manufacturers to entry into Japanese market.



.png)
.png)
.png)