Tokyo, Japan, June 16, 2020 - This serves as notification that JSR Corporation (Representative Director, CEO: Eric Johnson, hereinafter referred to as “JSR”) participated in OPEN COVID-19 DECLARATION. Based on the corporate mission of “Materials Innovation”, JSR will unite its technologies and knowledge and will work together to deal with the threat of COVID-19 infections.
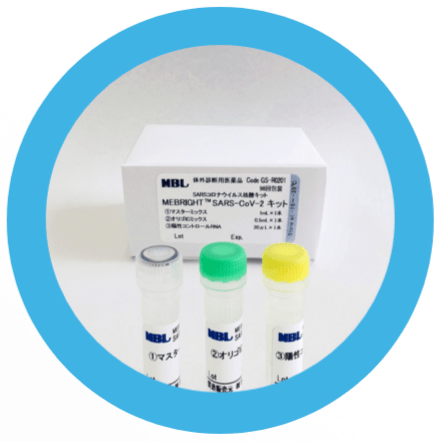
MBL began taking orders for a real-time PCR assay kit for the detection of the new coronavirus (SARS-CoV-2) on March 23.
Current testing kits require two individual assay steps to identify the two gene regions of SARS-CoV-2, while the MBL system can simultaneously detect these two regions in one single assay step by multiplex real-time PCR.
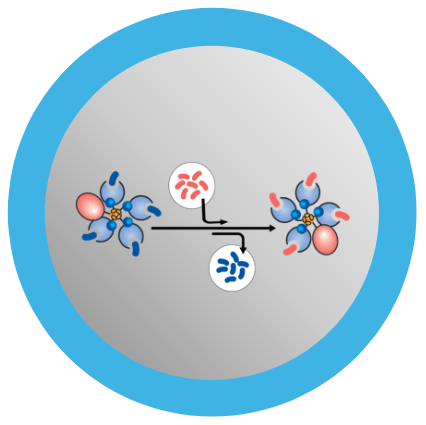
MBLI and the JSR Life Sciences team in Sunnyvale, CA have developed a COVID-19 T-Cell Peptide Screening Kit.
QuickSwitch Kits allow for the identification and validation of multiple SARS-CoV-2 protein epitope candidates that elicit immune responses, which can be used for vaccine development.
QuickSwitch also creates matched MHC tetramers to monitor T-cell responses to SARS-CoV-2 in blood samples.
KBI Biopharma is currently collaborating with existing clients to develop and manufacture therapeutic proteins and cell therapy treatments targeting COVID-19. Some of these treatments have already been clinically tested for other indications.
Regulatory agencies have granted fast track status for clinical evaluation of their efficacy against COVID-19. In addition, KBI received requests to determine the development strategy for ~12 new molecules to target the disease.